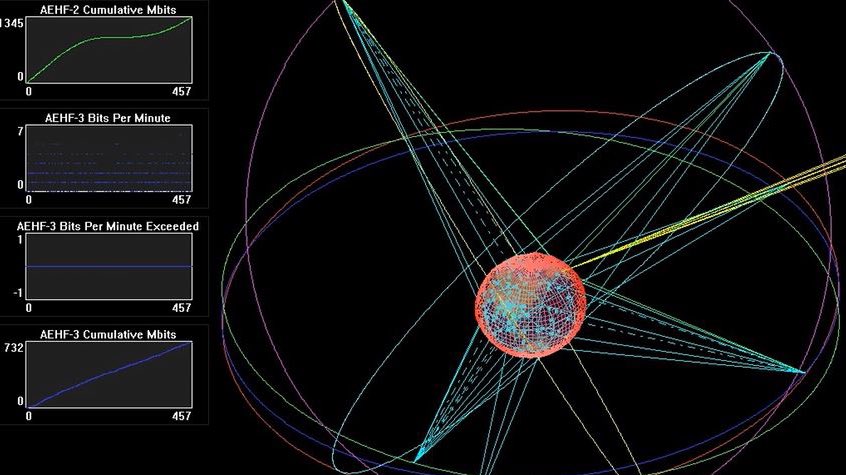
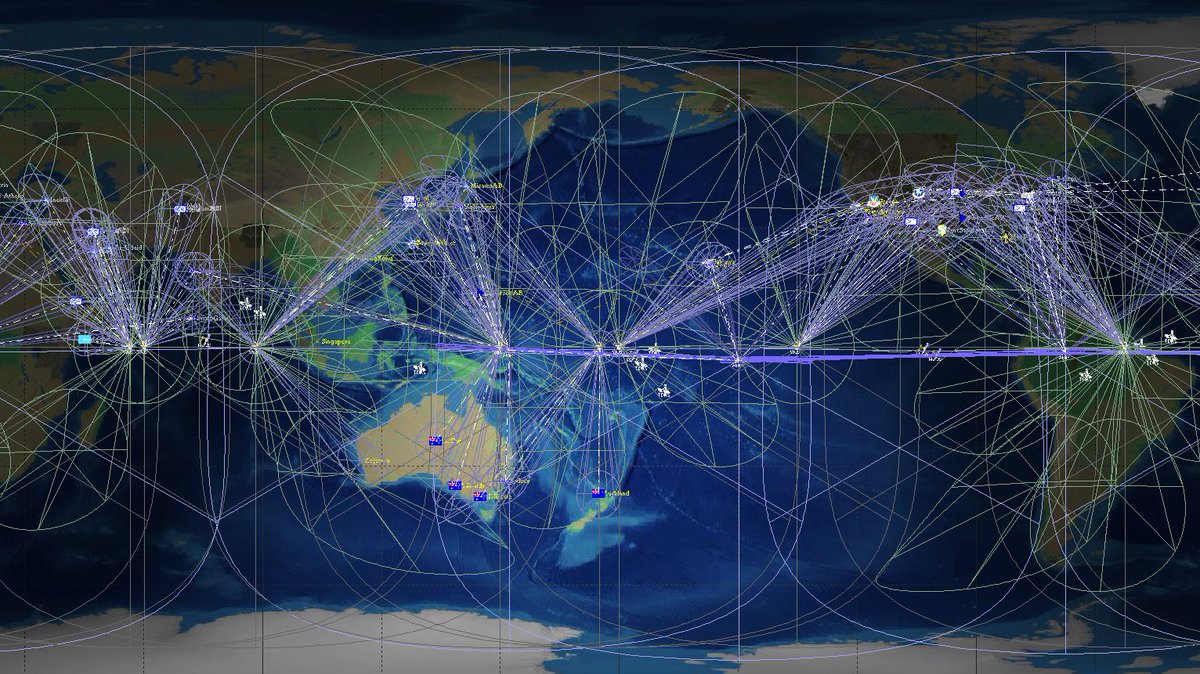
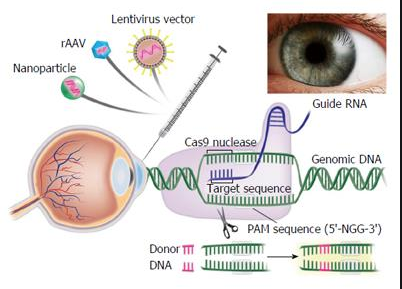
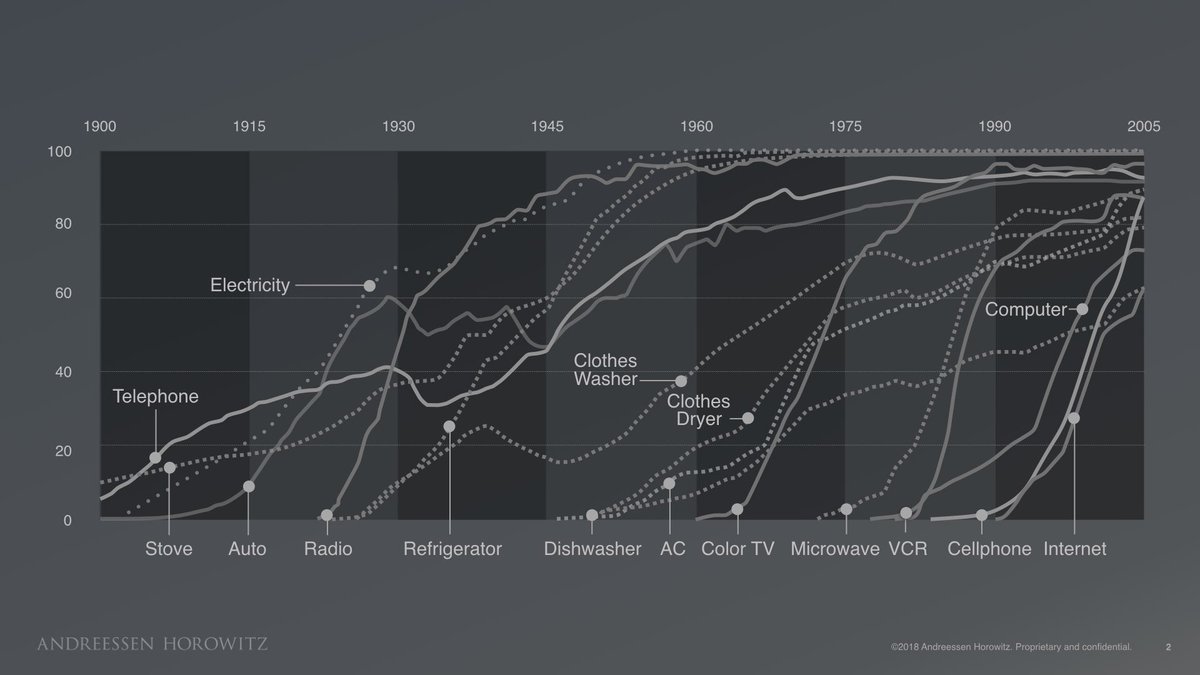
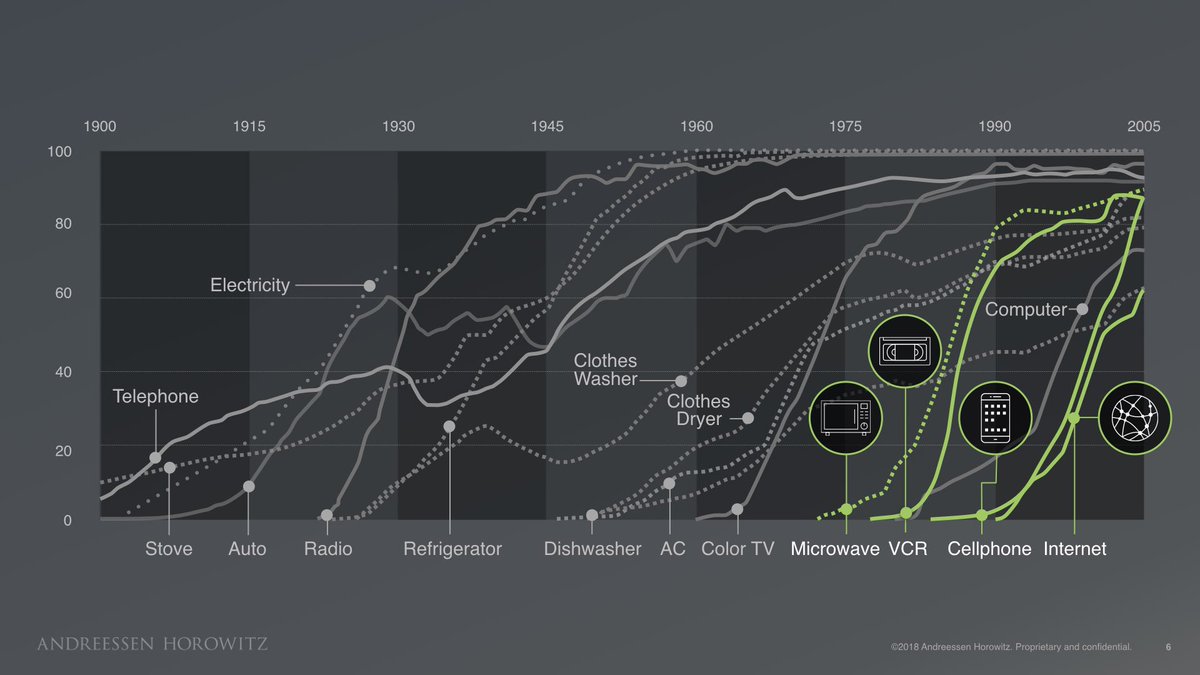
I am very late to this #MedicareForAll floor vote debate, but since it is still raging, I’ll join the fray by telling you a little story.

I've got some exciting news. @RepCarbajal just told our coalition that he will cosponsor #MedicareForAll!
— Ady Barkan (@AdyBarkan) June 28, 2019
I am grateful to him for taking this issue so seriously and doing whatever it takes to ensure that in America, health care really is treated as a human right! pic.twitter.com/5O5jZQ3LpS
More from Health
1/16
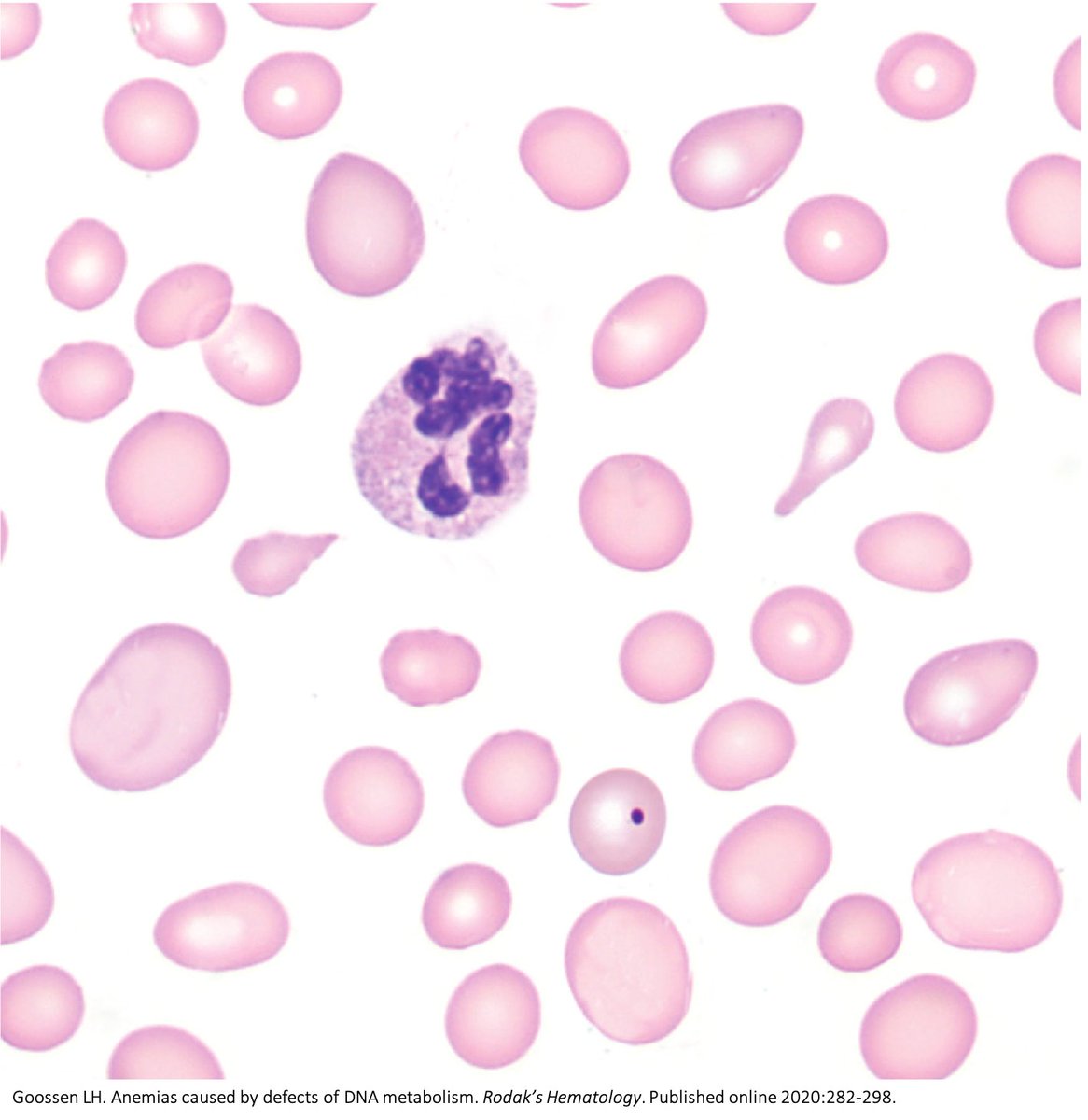
Why do B12 and folate deficiencies lead to HUGE red blood cells?
And, if the issue is DNA synthesis, why are red blood cells (which don't have DNA) the key cell line affected?
For answers, we'll have to go back a few billion years.
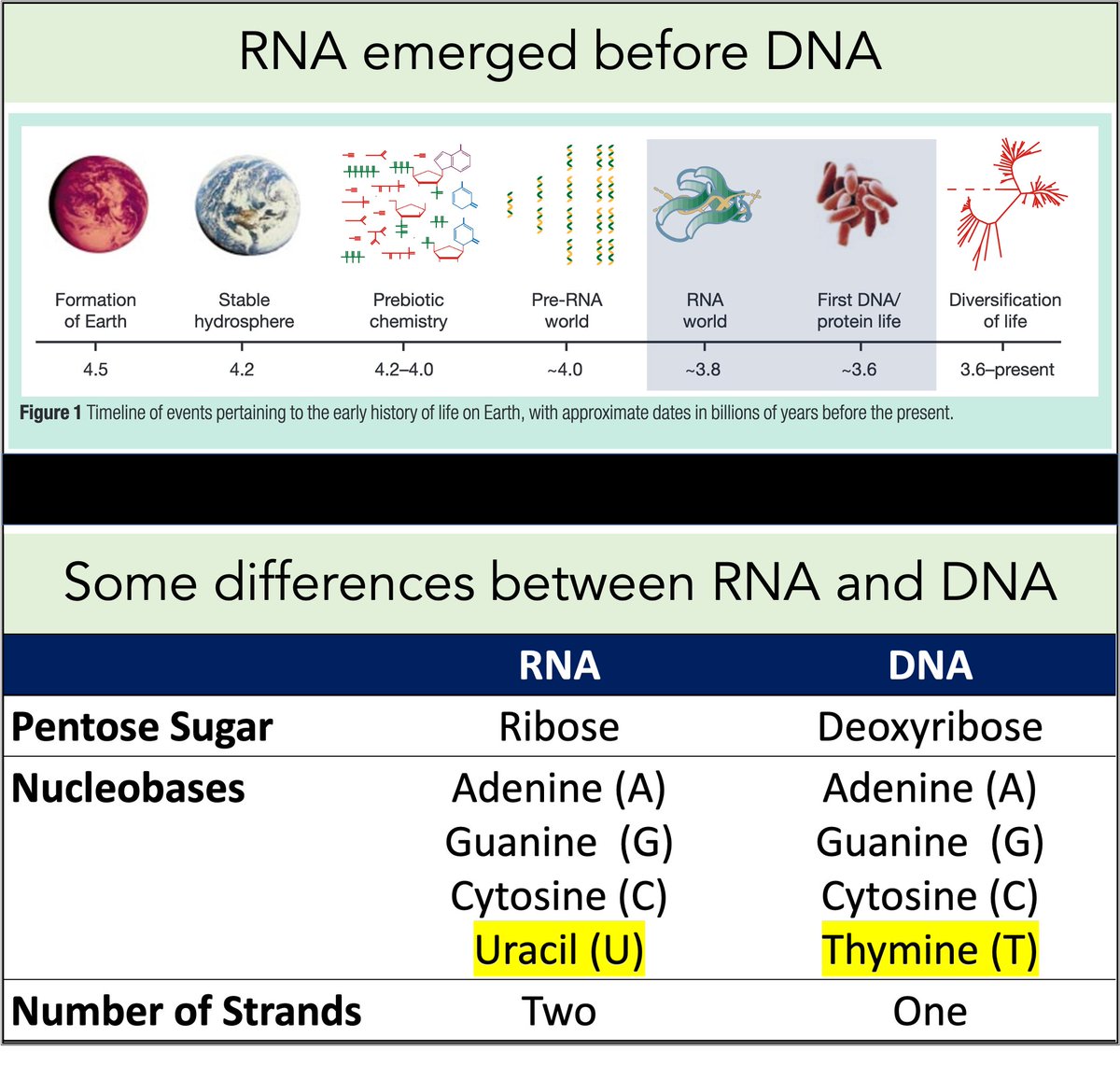
2/
RNA came first. Then, ~3-4 billion years ago, DNA emerged.
Among their differences:
🔹RNA contains uracil
🔹DNA contains thymine
But why does DNA contains thymine (T) instead of uracil (U)?
https://t.co/XlxT6cLLXg
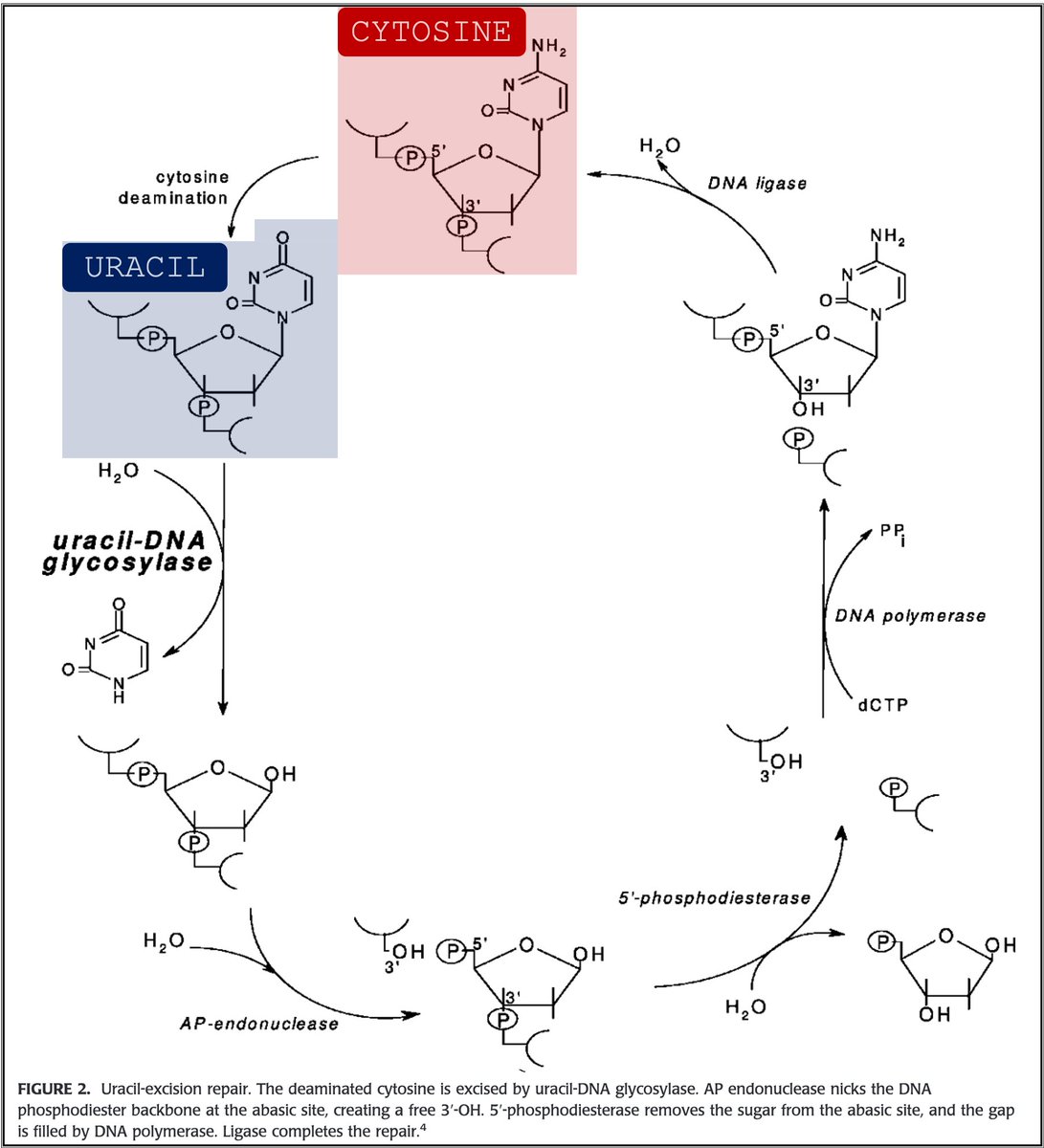
3/
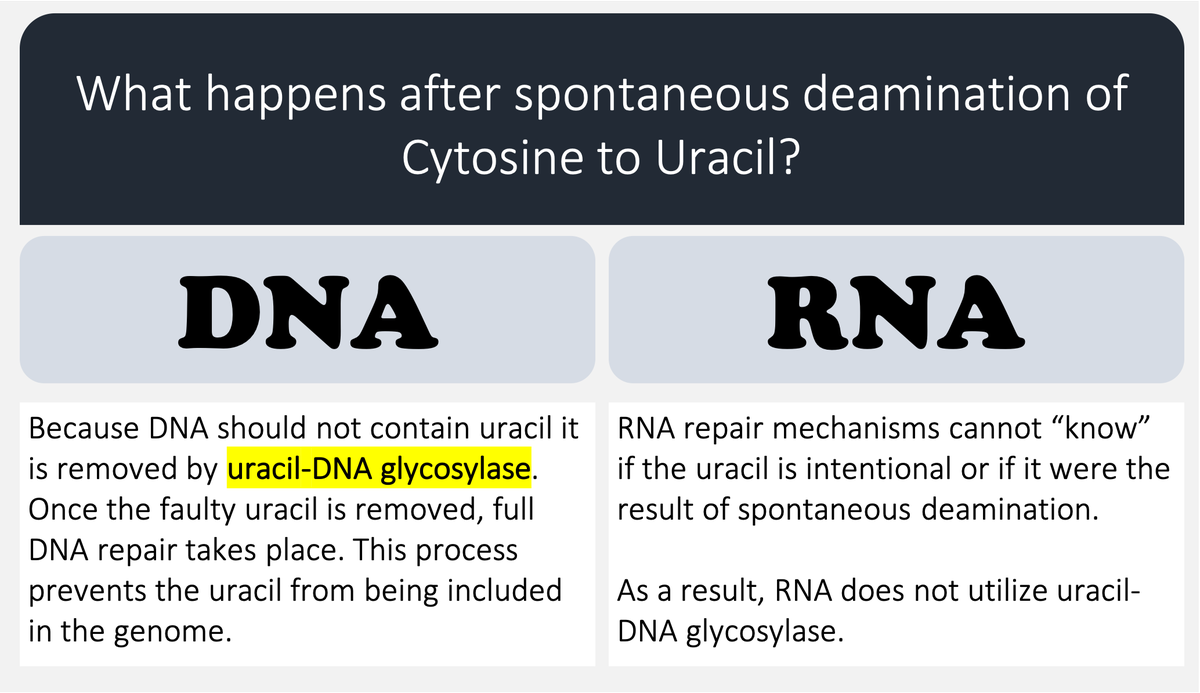
🔑Cytosine (C) can undergo spontaneous deamination to uracil (U).
In the RNA world, this meant that U could appear intensionally or unintentionally. This is clearly problematic. How can you repair RNA when you can't tell if something is an error?
https://t.co/bIZGviHBUc
4/
DNA's use of T instead of U means that spontaneous C → U deamination can be corrected without worry that an intentional U is being removed.
DNA requires greater stability than RNA so the transition to a thymine-based structure was beneficial.
https://t.co/bIZGviHBUc
5/
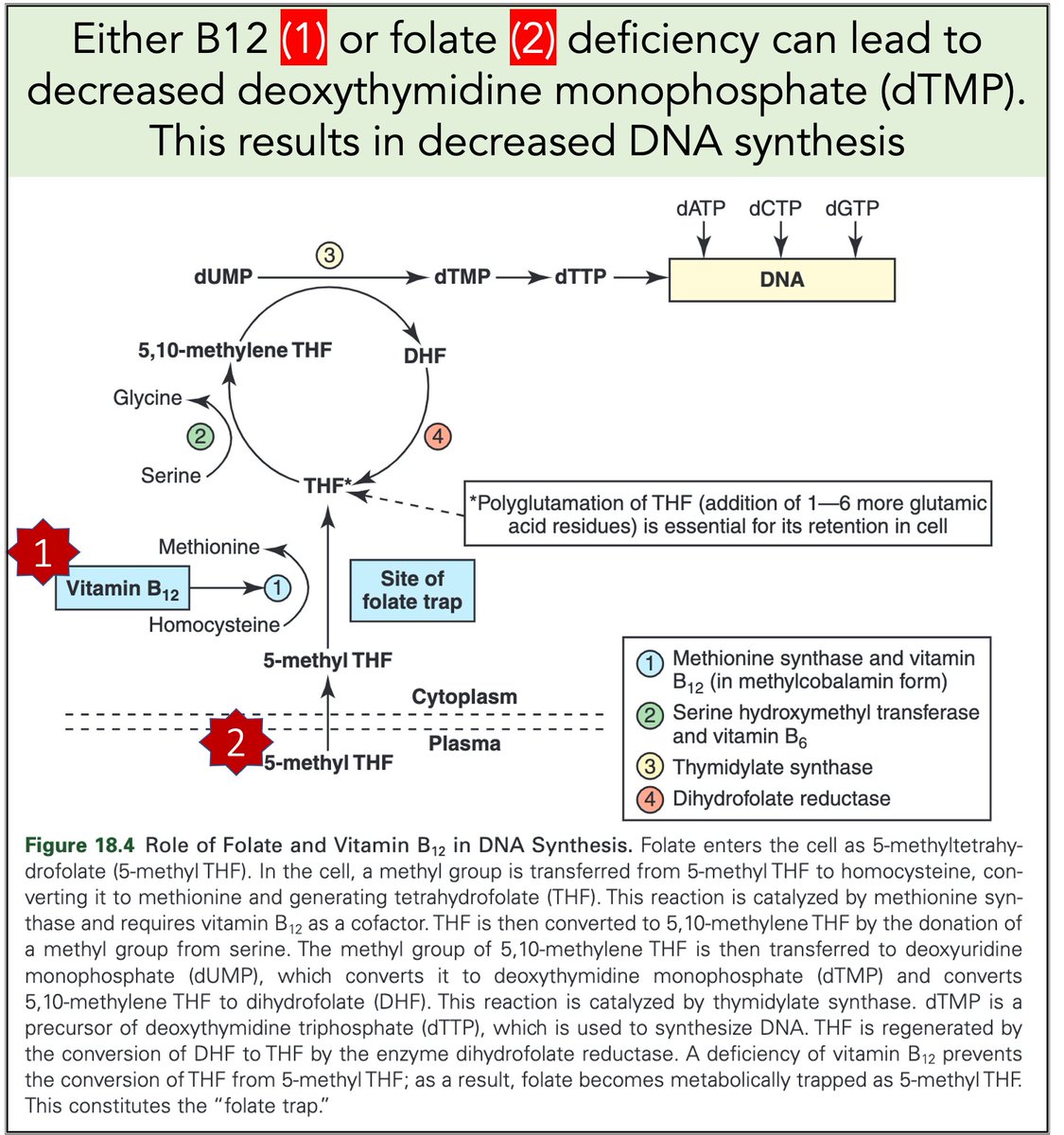
Let's return to megaloblastic anemia secondary to B12 or folate deficiency.
When either is severely deficient deoxythymidine monophosphate (dTMP*) production is hindered. With less dTMP, DNA synthesis is abnormal.
[*Note: thymine is the base in dTMP]
https://t.co/AnDUtKkbZh
Why do B12 and folate deficiencies lead to HUGE red blood cells?
And, if the issue is DNA synthesis, why are red blood cells (which don't have DNA) the key cell line affected?
For answers, we'll have to go back a few billion years.

2/
RNA came first. Then, ~3-4 billion years ago, DNA emerged.
Among their differences:
🔹RNA contains uracil
🔹DNA contains thymine
But why does DNA contains thymine (T) instead of uracil (U)?
https://t.co/XlxT6cLLXg

3/
🔑Cytosine (C) can undergo spontaneous deamination to uracil (U).
In the RNA world, this meant that U could appear intensionally or unintentionally. This is clearly problematic. How can you repair RNA when you can't tell if something is an error?
https://t.co/bIZGviHBUc

4/
DNA's use of T instead of U means that spontaneous C → U deamination can be corrected without worry that an intentional U is being removed.
DNA requires greater stability than RNA so the transition to a thymine-based structure was beneficial.
https://t.co/bIZGviHBUc

5/
Let's return to megaloblastic anemia secondary to B12 or folate deficiency.
When either is severely deficient deoxythymidine monophosphate (dTMP*) production is hindered. With less dTMP, DNA synthesis is abnormal.
[*Note: thymine is the base in dTMP]
https://t.co/AnDUtKkbZh

Sarcomeres in cardiac myocytes (heart muscle cells) are mechanically coupled to focal adhesions through dorsal stress fiber-like structures. #cardiotwitter #CellBiology
1/13
A thread based on Figure 1
A mature adult cardiac myocyte is packed with sarcomeres, whose contractile forces are coupled to the extracellular environment. With sarcomeres so close to the plasma membrane, how can we study the nature of this coupling?
2/13
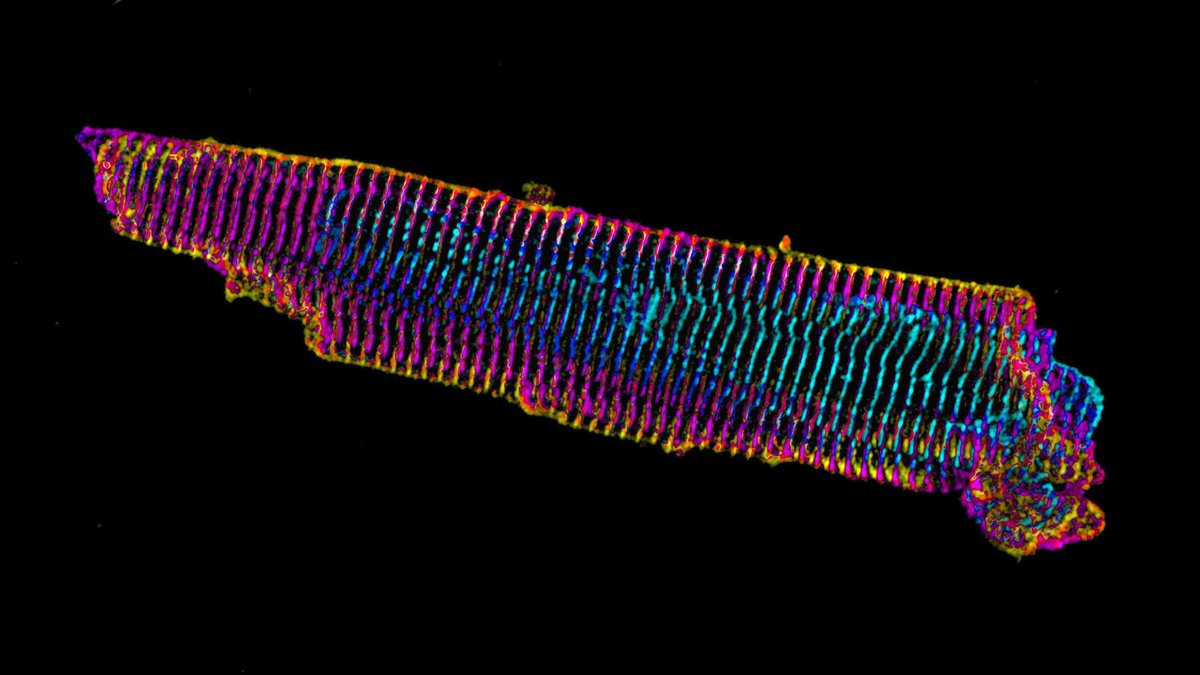
Short answer: find a model system where the sarcomeres are not so close to what the cardiac myocyte is attached to. Enter, iPS cell-derived cardiac myocytes. These are “immature” in culture as they resemble fetal or neonatal cardiac myocytes.
3/13
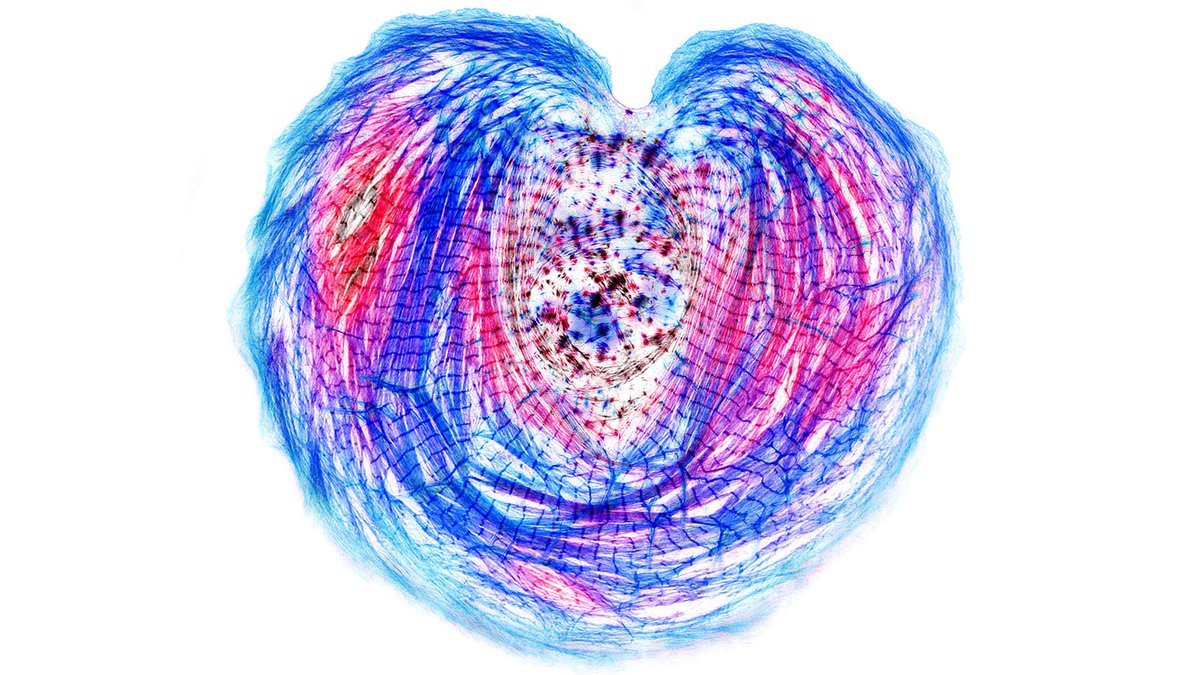
Our previous work on iPS cardiac myocytes reported that sarcomere containing myofibrils assembled on the top surface of the myocyte.
https://t.co/xIBCu3hG1W
4/13
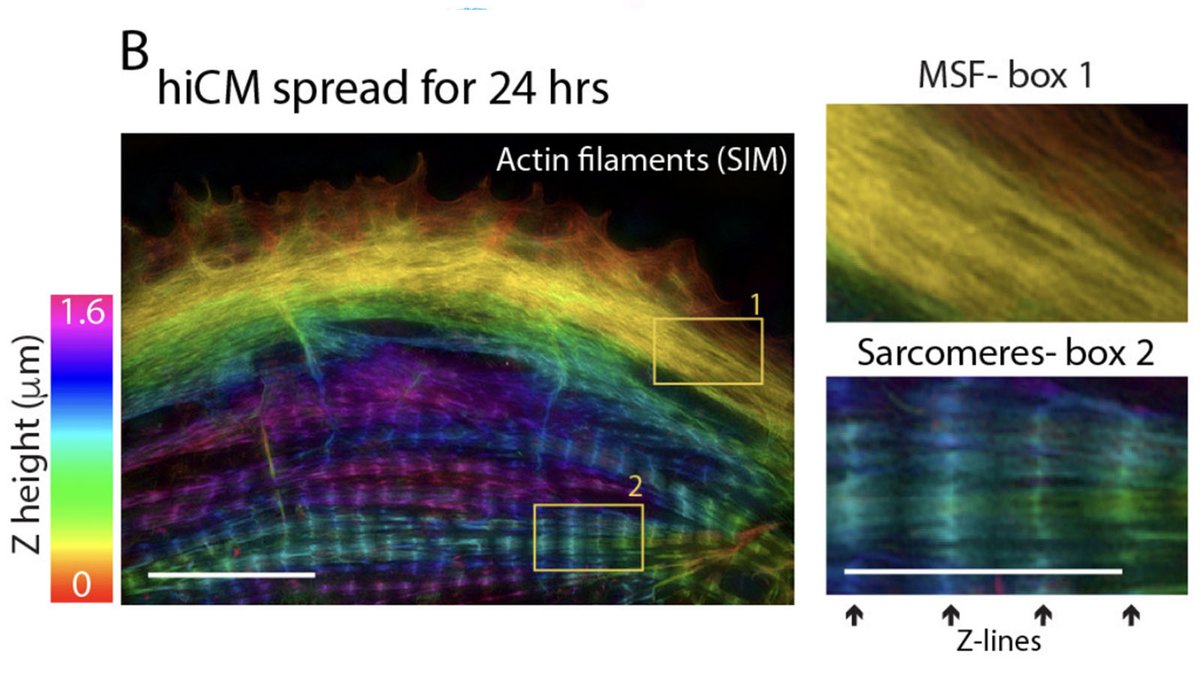
The sarcomeres seemed to be connected to focal adhesions on the bottom of the cell by thin actin bundles that resembled the dorsal stress fibers (DSF) commonly found in non-muscle cells. This movie steps through a Z stack of a myocyte starting at the bottom of the cell.
5/13
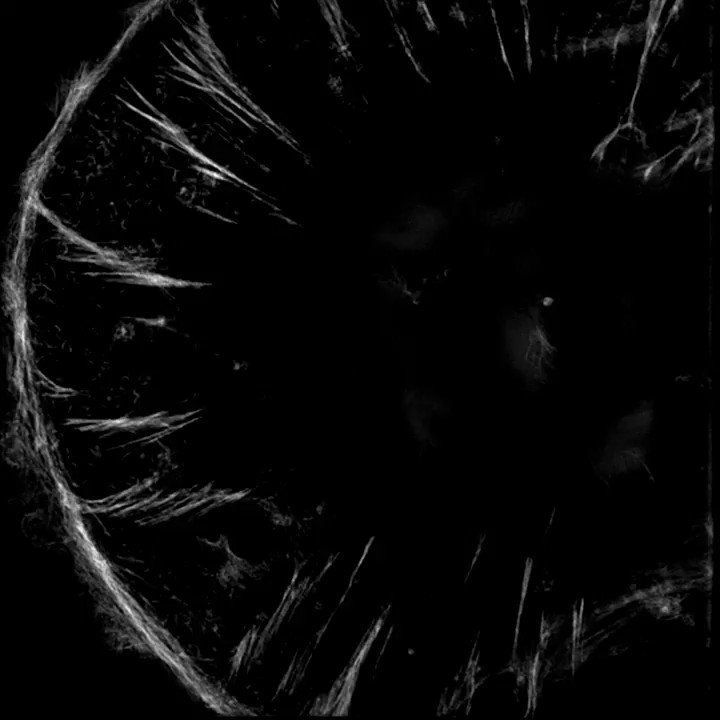
1/13
A thread based on Figure 1
A mature adult cardiac myocyte is packed with sarcomeres, whose contractile forces are coupled to the extracellular environment. With sarcomeres so close to the plasma membrane, how can we study the nature of this coupling?
2/13

Short answer: find a model system where the sarcomeres are not so close to what the cardiac myocyte is attached to. Enter, iPS cell-derived cardiac myocytes. These are “immature” in culture as they resemble fetal or neonatal cardiac myocytes.
3/13

Our previous work on iPS cardiac myocytes reported that sarcomere containing myofibrils assembled on the top surface of the myocyte.
https://t.co/xIBCu3hG1W
4/13

The sarcomeres seemed to be connected to focal adhesions on the bottom of the cell by thin actin bundles that resembled the dorsal stress fibers (DSF) commonly found in non-muscle cells. This movie steps through a Z stack of a myocyte starting at the bottom of the cell.
5/13