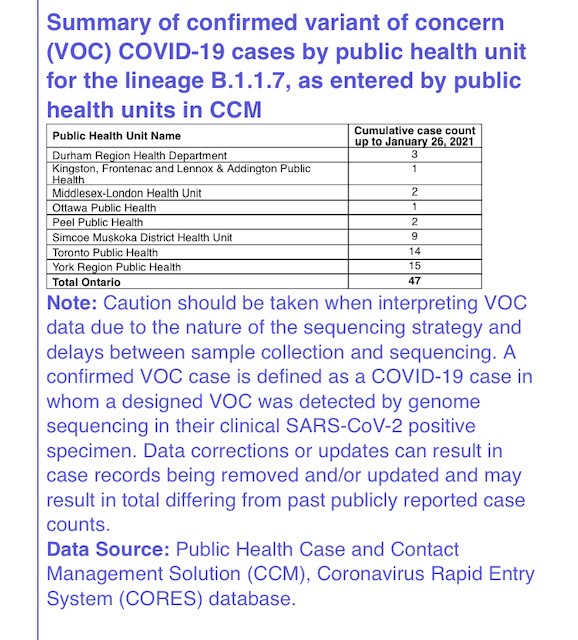
While #Tulane school of medicine is rightfully being exposed for its contemporary racism towards faculty, staff, students (and patients) due to the racist firing of Dr. Princess Dennar, it’s important to call out the deeply anti-Black history of this school, specifically... 1/
Tulane’s school of medicine rose to national prominence for its collection of human “specimens” in their anatomy museum “rivaled only by Harvard”. How did it acquire these “specimens” you might ask?
The collection was “curated” by a Dr. Edward Souchon... 3/
More from Health
🚨New lockdown regulations just published, in force tomorrow
The Health Protection (Coronavirus, Restrictions) (No. 3) and (All Tiers) (England) (Amendment) Regulations 2021
https://t.co/L5jwlTDaIE
(Thread)

These are not a new set of regulations: they are amendments an old set of regulations
Which we thought were gone! But they are back
Welcome back No.3 regulations
A quick thing before we continue!
I have been analysing these laws for free for 9 months now - if you want to say thanks and have a few £ to spare please give to my @LawCentres fundraiser
They give free legal advice to people who need it
They also amend the All Tiers regulations
Oh god it's all amendments by paragraph references
Basically all of England now in Tier 4 and Tier 4 is amended but not by a huge amount
This really is a terrible way to make laws on the fly - who can possibly understand it?!
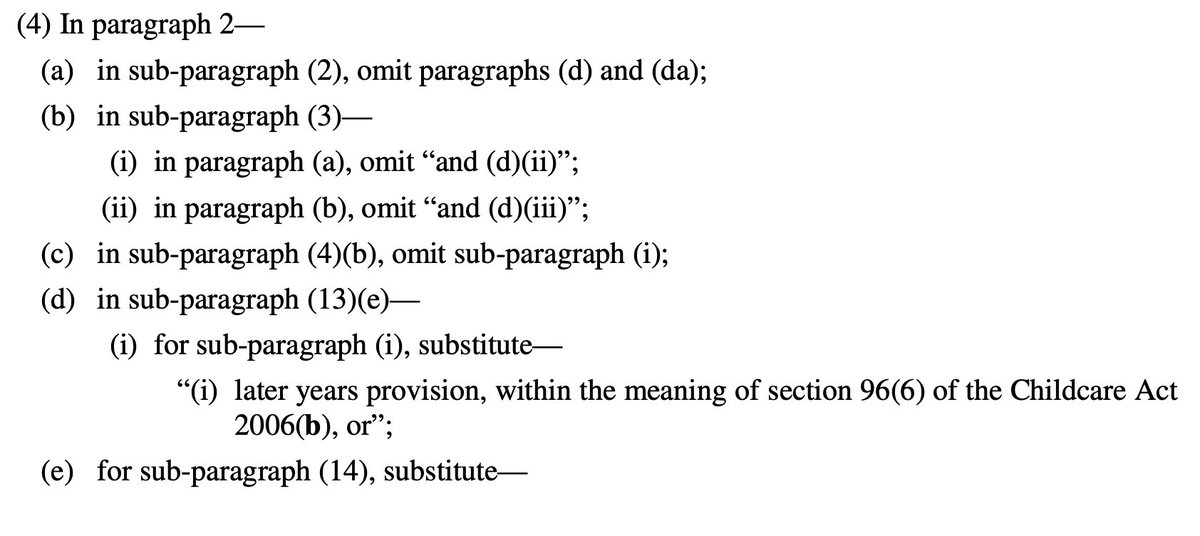
So, to explain, you need 2 documents open if you want to understand what is going on:
All Tiers regulations (Tiers 1-4, 2 December as amended) https://t.co/IraPQ112ak
And amendments https://t.co/L5jwlTDaIE
No sensible way of doing except by track changes, on it now, back soon
The Health Protection (Coronavirus, Restrictions) (No. 3) and (All Tiers) (England) (Amendment) Regulations 2021
https://t.co/L5jwlTDaIE
(Thread)

These are not a new set of regulations: they are amendments an old set of regulations
Which we thought were gone! But they are back
Welcome back No.3 regulations
A quick thing before we continue!
I have been analysing these laws for free for 9 months now - if you want to say thanks and have a few £ to spare please give to my @LawCentres fundraiser
They give free legal advice to people who need it
They also amend the All Tiers regulations
Oh god it's all amendments by paragraph references
Basically all of England now in Tier 4 and Tier 4 is amended but not by a huge amount
This really is a terrible way to make laws on the fly - who can possibly understand it?!

So, to explain, you need 2 documents open if you want to understand what is going on:
All Tiers regulations (Tiers 1-4, 2 December as amended) https://t.co/IraPQ112ak
And amendments https://t.co/L5jwlTDaIE
No sensible way of doing except by track changes, on it now, back soon