Authors Rajeev Venkayya MD
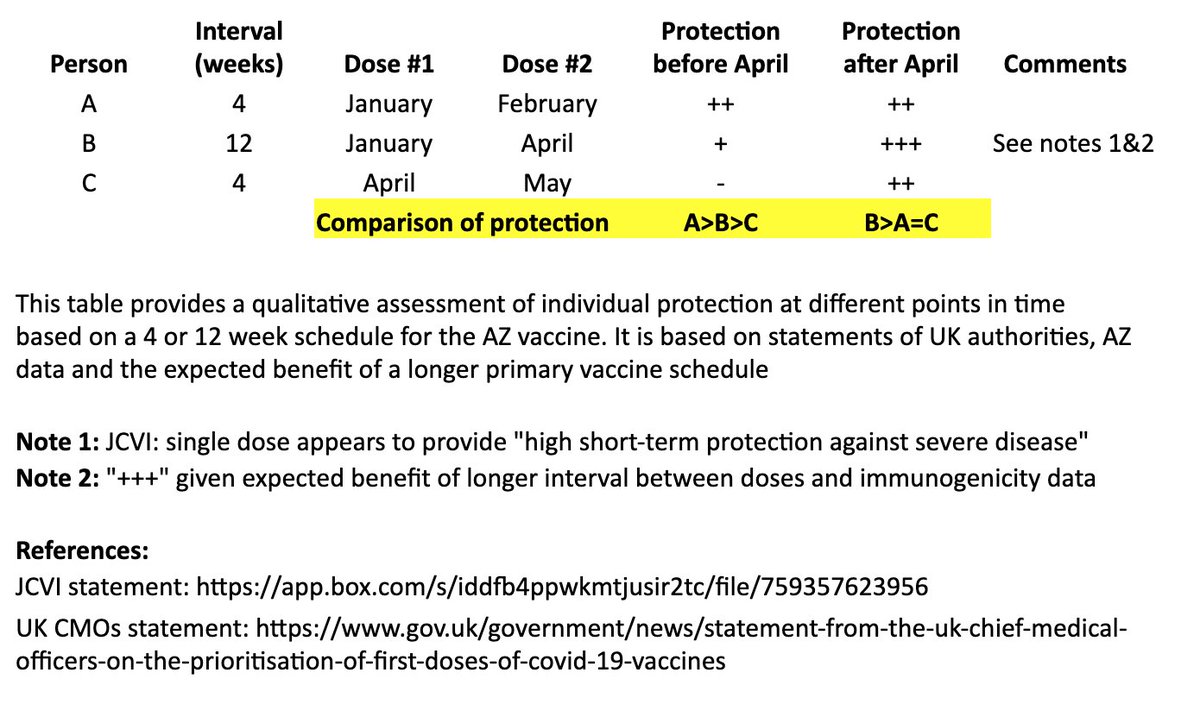
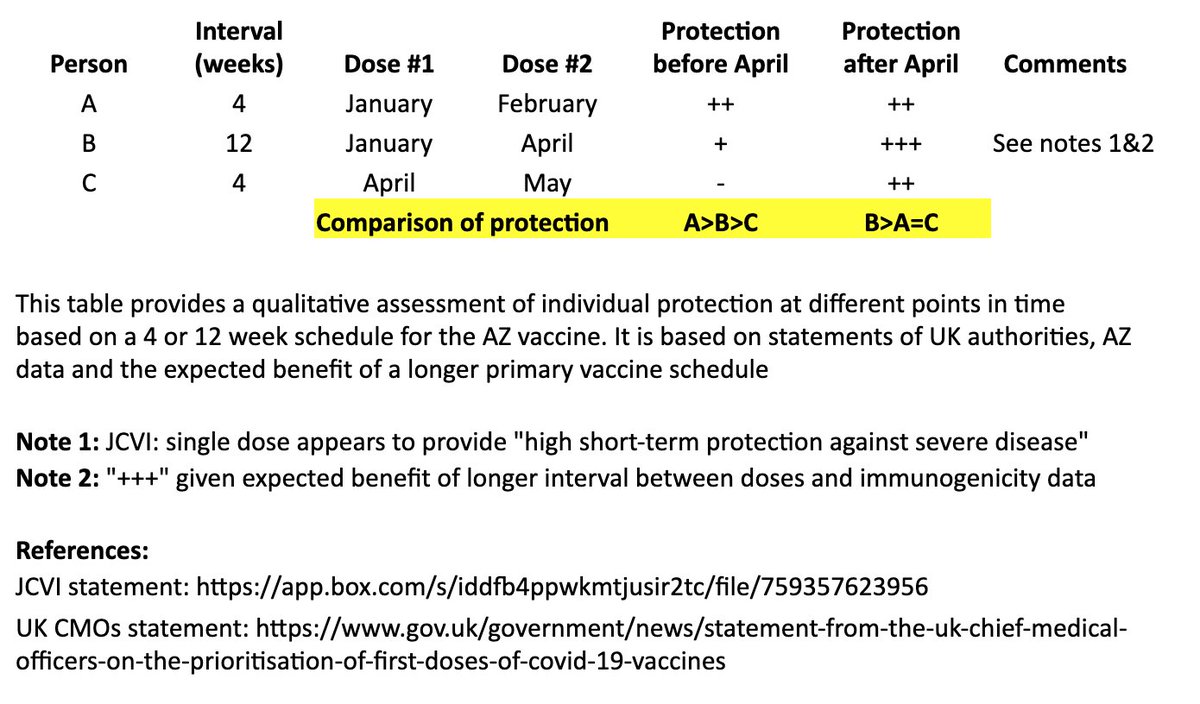
I created a simple table to illustrate the individual impact of the "flexible second dose timing" now recommended in the UK.
Coincidentally, @bob_wachter & @ashishkjha just tackled the US policy question in this important piece. 1/
https://t.co/n5bHkdIo0c
In @washingtonpost, @ashishkjha & I argue for the 2nd-shot-deferred strategy, partly by invoking the Mike Tyson principle. https://t.co/ZxrgVj3TJe We both came to this view because of the slow rollout & the new variant. But it's a tough call and reasonable people will disagree.
— Bob Wachter (@Bob_Wachter) January 3, 2021
I based this on recent statements from the UK chief medical officers, JCVI, and what we know from prior vaccine development. 2/
JCVI: https://t.co/6FQ25d6MFE
UK Chief Medical Officer (CMO) statement: https://t.co/RTpAIqgE1i
CMO letter to the profession:
This table and thread focuses on the AZ vaccine, where more data on a delayed second dose is available than with the Pfizer vaccine. It is not intended to address questions about single-dose regimens or mix & match approaches. 3/
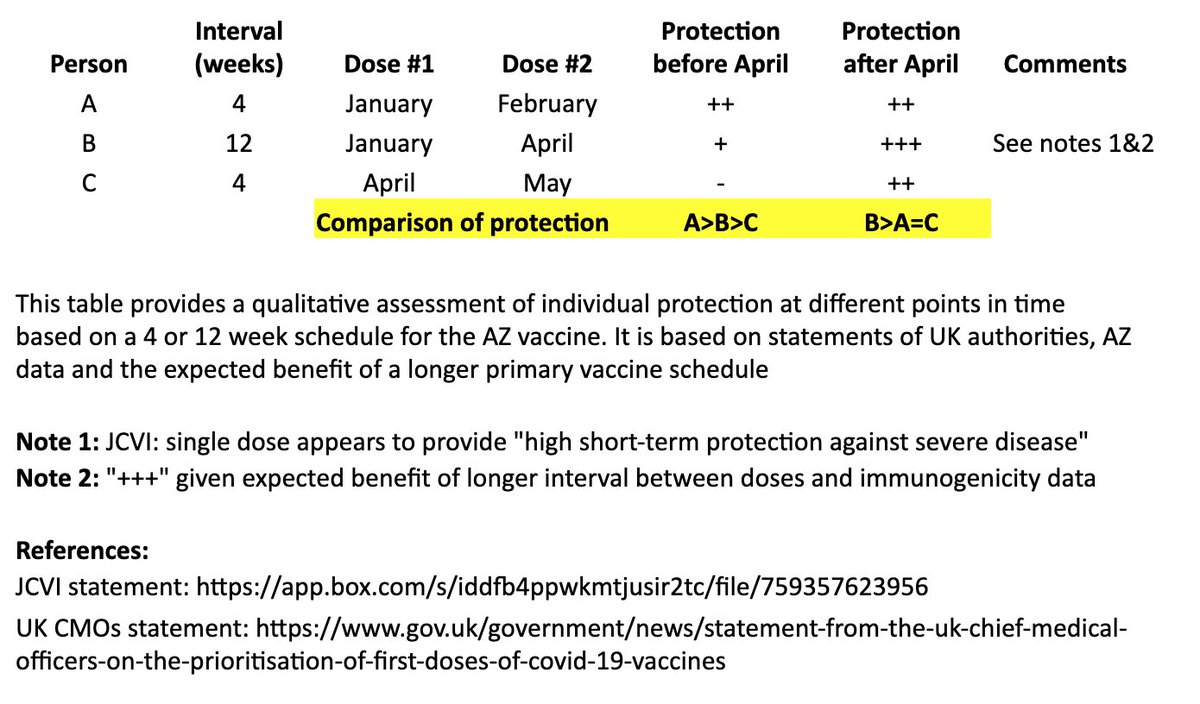
In the table, persons “A” and “B” both receive their first dose in January. “A” receives their second dose in February (4 weeks later), and “B” receives their second dose in April (12 weeks later). “C” receives their first dose in April and second dose in May (4 weeks later). 4/
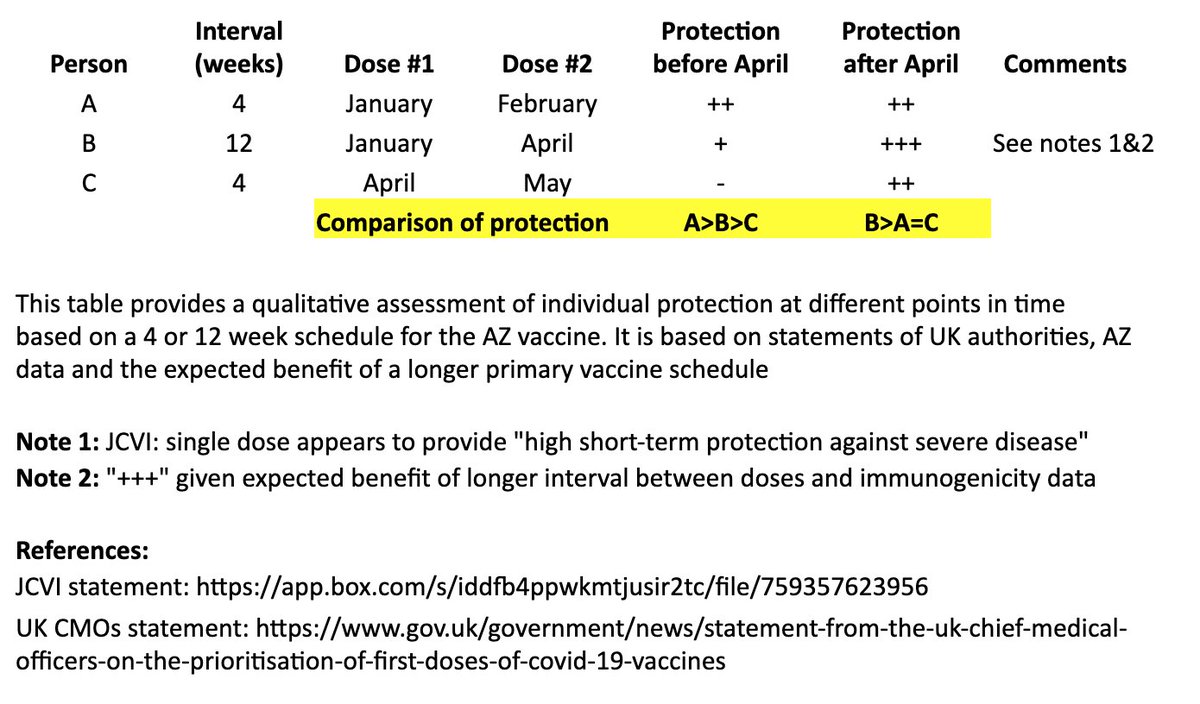
I made a qualitative comparison the potential efficacy during the two months between “A” and “B’s” second dose, as well as the potential longer-term efficacy after “B” receives their second dose. 5/
There’s much debate around the UK's recommended use of the AZ vaccine with a two-dose schedule and flexible timing of second dose. Some thoughts on the AZ recommendation (not Pfizer) based on available data with refs to some excellent threads.
1. It's a real pity the UK drug regulator, MHRA, doesn't hold public advisory committee meetings to assess #Covid vaccines, in the way @US_FDA does. Would have been fascinating to listen to a detailed analysis of the AstraZeneca-Oxford vaccine's emergency use application.
— Helen Branswell (@HelenBranswell) December 30, 2020
Thread
UK’s MHRA and JCVI are highly-experienced in vaccine assessments and recommendations, and they've surely weighed the benefits & risks of this recommendation carefully. That said, it would be good to see all the data underpinning their recommendation.
AZ\u2019s new claim is that they achieve 95% efficacy by increasing dose interval to 3 months. I see no efficacy data to support it in the new UK approval but it appears true that immunogenicity is much higher with that regimen (antibody titer nearly 3x higher than short interval) pic.twitter.com/ZtxSPjHUot
— Ed MD (@notdred) December 30, 2020
In general, vaccines should be taken on a schedule tested in an efficacy trial. But it wasn’t possible to conduct the typical dose and schedule optimization prior to these Ph3 trials, and those trials provided valuable data to inform these recommendations. 3/
The UK recommends a two-dose schedule, with the second dose between 4-12 weeks. This *is not* a single dose schedule. Given the data provided, and in the setting of limited supply, overstretched hospitals, and emergence of a more transmissible variant, this seems justifiable. 4/
The UK has important data on the AZ Vx that wasn’t available for Pfizer & Moderna at FDA's VRBPAC, including:
* single-dose efficacy through 4+ months; and
* single-dose immunogenicity (12+ weeks).
I've been seeing a lot of discussion around the dosage gaps recommended by government for the Astra/Oxford & Pfizer/BioNTech vaccines. My thoughts on the potential benefits & risks of such an approach, and the need for much greater transparency around these decisions. Thread. pic.twitter.com/mclqMDeMQ1
— Deepti Gurdasani (@dgurdasani1) December 31, 2020
